Posted 8:32 a.m. Friday, Sept. 27, 2024

UWL scientists explain the basic science behind the beauty
As the vibrant hues of autumn approach, the fall 2024 leaf color display in Wisconsin is likely to start later than usual, thanks to an unusually warm September, predicts Adam Schneider, an assistant professor at UW-La Crosse.
“Given the warmer September temperatures, I think we will probably see later than normal peak color,” Schneider explains. However, he emphasizes that the amount of rainfall during October will be crucial in determining the quality of this year's display.
“If we receive a modest amount of rain, we could be in for a spectacular show,” he notes. “Conversely, if conditions remain dry and plants experience stress, the colors would become more muted.”
The ideal conditions for a stunning fall display consist of sunny, warm days paired with cool nights and sufficient moisture. More rain generally promotes vibrant colors by helping trees maintain their health and boosting sugar production. However, sudden heat waves or dry spells can dramatically impact the foliage, Schneider warns.
In a typical year, La Crosse County experiences peak leaf colors around mid-October. According to the Travel Wisconsin Fall Color Report, the brilliant display of fall leaves is set to peak throughout October across the state.
Why do leaves change color?
Leaf color change is determined by several factors, which is why trees change at slightly different times during the fall season. Schneider and UWL Chemistry Professor Heather Schenck point to several factors that influence leaf color:
- Pigments inside the leaf - Production of chlorophyll molecules that dominate the leaf during summer months stops, revealing yellow and orange molecules present in the leaf.
- The shortening length of fall days - Trees know when days are getting shorter and nights are getting longer and they respond by slowing and then stopping the production of chlorophyll molecules.
- Weather conditions - The best conditions in the fall for leaves are warm and sunny days, followed by cool and crisp but not freezing nights, according to the U.S. Forest Service.
- Soil conditions - Dry, sandy soil will lead to a less brilliantly colored leaf.
- Tree health - Trees that are older or unhealthy tend to change sooner.
Some of these conditions — namely soil, weather and tree health — vary from year to year, causing slight changes to the length of the fall foliage display and brilliance of the leaves each year.
Below Schenck answers other common science questions related to fall foliage.
What causes fall leaves to change color?
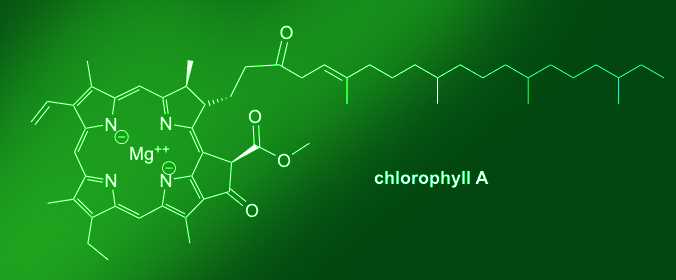
We are used to seeing green leaves throughout the summer months. If you were to shrink down and look inside green leaves, you’d see that they are swamped with molecules that make it look green —a.k.a. chlorophyll molecules. Chlorophyll has an important job inside a leaf. It takes light from the sun and converts it into energy that the tree uses to grow through a process called photosynthesis. Chlorophyll breaks down in sunlight, so throughout the summer this molecule is continually being remade.
In the fall, in the north, we have less sun during the day and trees slow and then stop producing chlorophyll molecules. When this happens, other molecules that were already inside the leaf — just not as prominent as chlorophyll — begin to appear. Basically, leaves are chemically revealing other molecules already there, but we couldn't see them because the leaf was so filled with chlorophyll.
Why do leaves turn different colors?
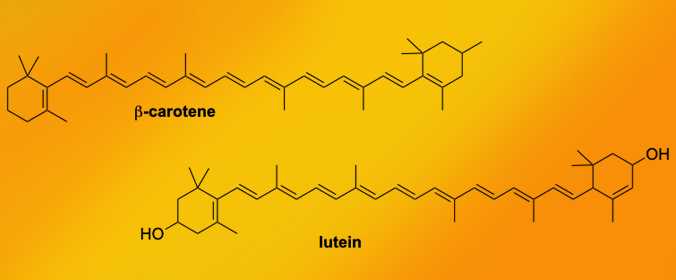
Other molecules besides chlorophyll can give color to a leaf. In fact, there are so many other color molecules that they are named in classes. A common class of molecules that give leaves a yellow-orange color are called carotenoids. Carotenoids can help plants gather more light in an energy range that chlorophylls don't pick up well.
Another class of molecules that make a leaf look red or purple are called anthocyanins. These color molecules also show up in our foods. You may ask: Why are carrots orange? Carotenoids. Why are apples red? Anthocyanins.
What determines leaf color?
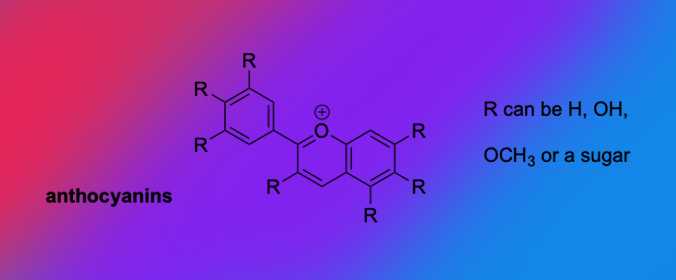
The other color molecules — anthocyanins and carotenoids — are a more diverse group than chlorophyll. Therefore, we see a spectrum of different colors. For example a black maple might produce more of one type of color molecule, making it look a deep orange. A different black maple might produce less of that molecule and more of another, making it look yellow. It is the concentrations of these more diverse molecules that can make a tree a certain color or even different colors throughout the same tree or the same leaf.
Although scientists understand how leaves change color at the molecular level, they still haven't come to a consensus on why some trees turn red (or why the new growth of some plants is also reddish). Here are two hypotheses:
- As chlorophyll is being lost, anthocyanins help prevent the leaves from becoming sunburned before plants have a chance to fully recycle their nutrients.
- The red color of anthocyanins makes leaves less palatable to insects or herbivores.
What makes fall leaves more colorful?

- Moist spring
- Summer that's not too hot or dry
- Warm and sunny late summer and early fall
- Fall nights that are cool but not freezing
When is peak leaf color change in Wisconsin? Fall foliage map 2023.
The color change in Wisconsin typically starts in mid-September and ends in October. The northern part of the state will begin to see change before the south. The La Crosse area is estimated to peak the second week in October. Check out Fall Color report will give you the estimated week of the peak in your area.
Is climate change having (or may it have) noticeable effects on when or where colors appear?

Compared to the 1950s, Wisconsin weather is three degrees warmer and 15% wetter. While three degrees may not seem like much, it means that it takes an extra week in the fall for plants to start experiencing cooler temperatures. Both moisture and warmer temperatures, especially at night, tend to delay the onset of fall color. Also, the overall increased variability of rainfall over the last fifty years — flash floods interspersed by long droughts — has a big effect on tree health and thus the length and color of the fall display.
While we may be experiencing wetter years averaged over time, the last three years in Wisconsin have brought less-than-average rain in summers and somewhat lower rainfall totals in the fall as well. The dry spell in summer 2023 was the most intense of the past three years in many areas of the state.
Listen in to Wisconsin Public Radio's The Morning Show featuring UWL Biology Professor Tim Gerber and UWL Chemistry Professor Heather Schenck sharing the science behind why fall leaves change color.
