Human subjects review Institutional Review Board (IRB)
A page within Research & Sponsored Programs
Note: Due to the Graff Main Hall HVAC renovation project, the IRB central office is now temporarily located in 339 Wimberly Hall.
Help With Human Subjects (IRB) Review
The Associate Vice Chancellor for Academic Affairs provides administrative oversight to the Institutional Review Board (IRB) for the Protection of Human Subjects, its Executive Committee, and its chair. The IRB's function is to review research protocols for the use of human subjects in research (funded or non-funded) proposed by faculty, staff, and students of UW-La Crosse.
A guide and necessary forms for submission of proposals to the IRB are available from the links below. The office is in 243 Graff Main Hall and can be contacted via phone at 608.785.8044 or email at irb@uwlax.edu.
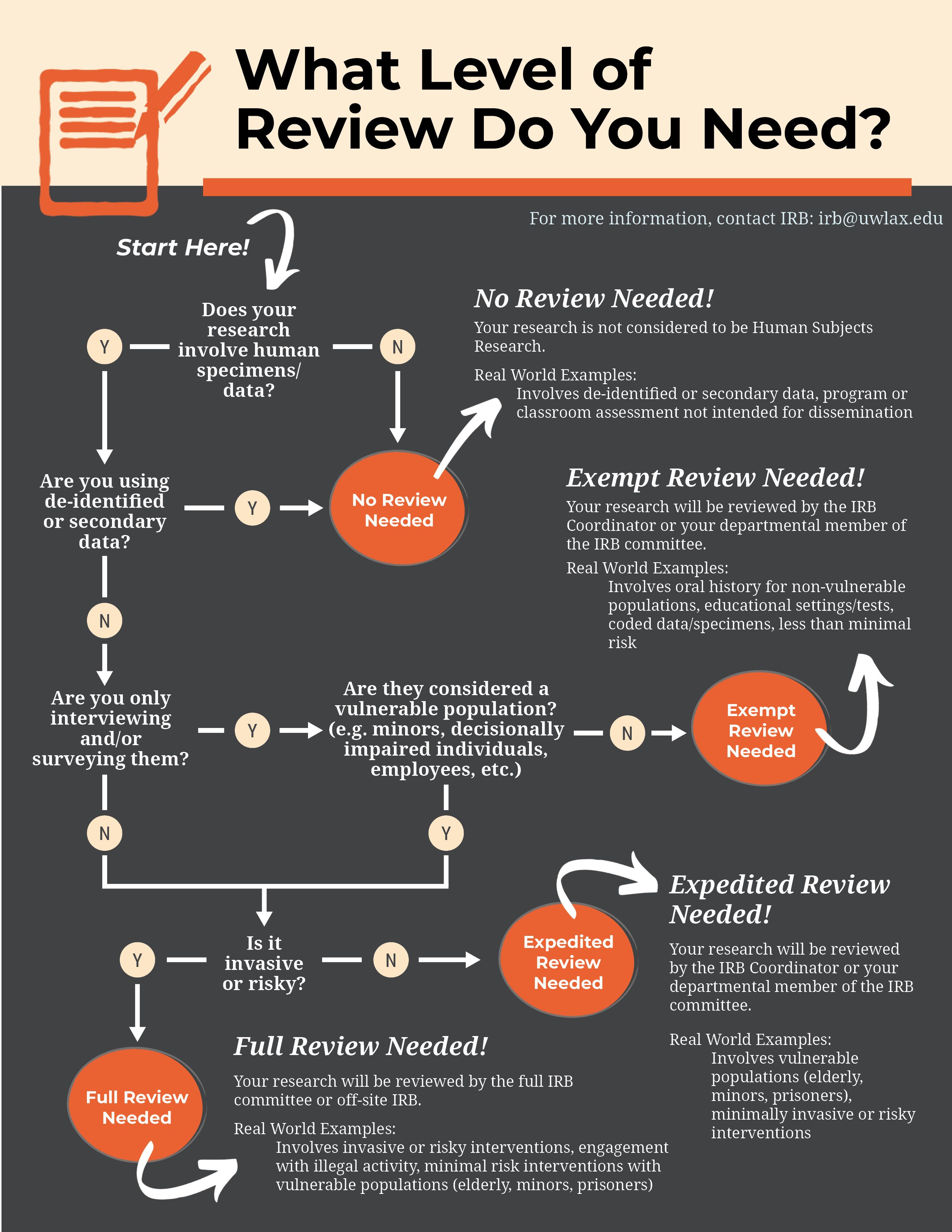
Protocols are categorized as either exempt, expedited, or full - see the Researcher's Guide for Submission of Protocols and the IRB Decision Map to assist you in determining into which category your protocol falls.
If you believe your protocol is exempt after consulting the Researcher's Guide, please see the "Exempt Decision Tool" information below. Note: Not all "exempt" protocols will qualify for review with the tool.
All other protocols must be submitted electronically to irb@uwlax.edu. All items of the Narrative Statement must be numbered to correspond to each specific IRB requirement. Forms with signatures must be scanned and electronically inserted into your submission document. All materials should be submitted in one continuous PDF file. Complete instructions can be found in the Researcher's Guide for Submission of Protocols.
Required Online Training Course
Registering for a CITI Program Training Account & Human Subjects Research Courses
Note: If you already have a CITI account and just need to add the Human Subjects Research course, log in and then select "Add a Course". Skip to Step 7 of the instructions below to enroll in the Human Subjects Research training program.
Step 1: Go to the CITI registration page and under “Select Your Organization Affiliation”, type “Wisconsin” in the box and then select University of Wisconsin – La Crosse. Click on “Continue To Create Your CITI Program Username/Password”.
Step 2: Enter your first and last name and email address. Enter your name here as you would like it to appear on your completion reports. *Please note that using your UWL-issued email address is preferred for account recovery purposes.* Click on “Continue to Step 3”.
Step 3: Choose a username, password, and security question. Click on “Continue to Step 4”.
Step 4: Enter demographic information and click on “Continue to Step 5”.
Step 5: Click “NO” when asked if you want to request Continuing Education Unit (CEU) credits. Answer the next two questions and click on “Continue to Step 6”.
Step 6: Complete the personal information requested. For your Department, students can use their major (Biology, Microbiology, etc.). For the Role in Research, choose a role that is most appropriate for your involvement in the research. Click on “Continue to Step 7”.
Step 7: Scroll down to Question 7 (SKIP ALL OTHER QUESTIONS FOR TRAINING), “Human Subjects Research” to select your curriculum*. Choose one learner group from the list below based on the type of human subjects research activities you will conduct:
- Biomedical Research Investigators: Choose this group to satisfy CITI training requirements for Investigators and staff involved primarily in Biomedical research with human subjects.
- Social & Behavioral Research Investigators: Choose this group to satisfy CITI training requirements for Investigators and staff involved primarily in Social and Behavioral research with human subjects.
- Research with data or laboratory specimens- ONLY: No contact (e.g., surveys) with human subjects. This training covers the use of identifiable secondary data. If you or your research team are collecting data, the appropriate training would be either the Biomedical or the Social & Behavioral training.
- IRB Members: This Basic Course is appropriate for IRB or Ethics Committee members.
* If you are not sure which category you fit into, please check with the IRB office or your faculty advisor.
Step 8: Click on “Submit” at the bottom of the page.
Instructions for Completing the Required CITI Human Subjects Research Training Courses
Step 1: From your list of courses, select the IRB course you’ve recently added to begin (Biomedical Research, Social & Behavioral Research, Biomedical Data or Specimens Only Research, or IRB Members)
Step 2: Complete the Integrity Assurance Statement presented at the top of each course. The system will allow you to start taking the course modules after completing it.
Step 3: Click the title of each course to begin or continue the course.
Step 4: Complete the required modules and associated quizzes. Please read the course main page for requirements, as there may be elective courses required based on your selected course. Supplemental modules are optional, unless assigned by a professor, a supervisor, or a supervising investigator, or required of you by the IRB due to the nature of your project.
The minimum "passing" aggregate score for the quizzes is 80%. A running tally is compiled in the grade book. If you want to improve a score on a quiz, you may repeat any quiz. Scores obtained after a completion report has been issued will not be reflected on the completion report.
Step 5: When you complete all required modules of the course successfully, a Completion Certification will be available for you. The IRB will need a copy of this document included in your IRB Application. You can access it under the “My Records” tab.
Find the IRB course you have just completed, and click “View-Print-Share” under “Completion Record”. There will be two options for completion records – the standard document needed can be found by clicking the link for the Completion Certificate.
Step 6: Save this Completion Certificate as a PDF or Word document, and include it in the last page in your IRB Application.
Note: if supplemental modules have been required of you (see Step 4), you should ALSO save and attach the Completion Report that is available on the same page as the completion certificate.
General questions and password reset requests should be addressed to irb@uwlax.edu
Technical issues with the CITI web site should be addressed to support@citiprogram.org or to 888-529-5929.
For more information about the required IRB training course and certification, please see the IRB Training FAQs at the bottom of this page.
IRB Guidelines and Forms
Researcher's Guide for Submission of Protocols
Researcher's Guide for Submission of Protocols
Note: conducting research outside of the United States does not eliminate your research compliance (e.g., IRB, etc.) obligations. Usually these approvals must be received before finalizing your trip. Contact irb@uwlax.edu for assistance with determining what, if any, approvals or agreements you may need.
IRB Noncompliance Policy
Please refer to the following policy document for information on noncompliance in human subjects research and how to request a retrospective review: IRB Noncompliance Policy_final.pdf
If you would like to report what you believe to be an instance of noncompliance for research involving human subjects, or have concerns that your own research may be in noncompliance, please email irb@uwlax.edu or fill out the IRB anonymous Qualtrics survey.
COVID-19 and Human Subjects Research
This is to provide general guidance about research during this time of COVID-19.
An IRB protocol or other research procedures may NOT be used as a reason to bypass or subvert state, local, or university safety requirements.
- Masks must be worn.
- Maintain a distance of at least 6 feet. If this is not possible, closer contact should not exceed 15 minutes.
- Sanitizer must be available.
- Additional protocols required in individual labs (e.g., using hand sanitizer upon entering or exiting a lab) must be followed.
For those interested in training related to COVID-19, CITI offers optional training titled "COVID-19: Back to Campus (Fall 2020)" which includes an optional module titled "COVID-19: Human Subjects Research".
IRB Exempt Decision Tool
This tool is for UWL faculty, staff, and students to seek an exempt status designation for a research project involving human subjects. Exemptions are only valid if requested by and granted to a current UWL employee or currently enrolled UWL student.
Important Notes:
- Students must have mentor/advisor approval before using this decision tool.
- The tool may NOT be used if any of the following are true:
- Participant incentives are being offered
- Any of the PIs are not UWL-affiliated
- Other institutions will be relying on UWL's IRB to provide review and oversight (See "Single IRB / Reliance Agreements" in the Researcher's Guide)
- Exemption is being claimed under exemption research categories numbered 2.c., 3.a.iii., 7, and/or 8
Prior to completing this survey, you should:
- complete the mandatory CITI training;
- review the Researcher's Guide for Submission of Protocols to ensure that your research meets the definition of exemptible research;
- review the IRB Exempt Decision Tool Preview to ensure you are able to answer the questions asked; and
- have the following information available to complete the survey:
- names and email addresses of any Advisors or co-PI/PDs;
- IRB training completion certificate(s) from a UWL approved course for you and any co-PI/PDs (collated into a single pdf with your certificate listed first);
- project title
- project abstract which must include the research question, independent and dependent variables, hypothesis(es), and procedures and/or activities that the subjects with undergo; and
- basic information about the project
- see the Researcher's guide for optional information that you may choose to include
DO NOT GUESS on any of the answers in the survey. If you are unsure how to answer a question, reach out to your advisor, colleagues, or the IRB, and return to complete the survey.
After completing all or part of the Exempt Decision Tool, you will receive an email either declaring your project, as described, as exempt or directing you to submit a protocol narrative to the irb@uwlax.edu for review.
Question and concerns regarding the form/survey should be directed to irb@uwlax.edu.
Attachment Templates
- Attachment A - Application for University IRB Review
- Attachment B - IRB Determination Form (for Federal Funding Agencies)
- Attachment C - UWL IRB Revision or Report Form
- Use to request approval of:
- protocol changes (exempt, expedited and full)
- consent form changes (expedited and full)
- Use to report
- study closure (full)
- end date changes: early closure or extension of the end date (expedited)
- change in lead PI/PD (expedited and full)
- adverse event(s) (expedited and full)
- annual continuing review (full only)
- Use to request approval of:
- Attachment D - Waiver of Informed Consent Application
- Use to request approval of:
- waiver of informed consent for participants 18+ years of age who are otherwise capable of consent
- waiver of informed parental/legal guardian consent for minors or those 18+ years of age with legally appointed decision-makers
- Use to request approval of:
Informed Consent & Debriefing Examples
Operating Guidelines and Organizational Policies
Unanticipated Problems & Adverse Events
Human Health Services (HHS) requires that all non-exempt human subjects research conducted by UWL faculty, staff, and/or students report if their study results in any Unanticipated Problems (UPs).
See the IRB Adverse Events & Unanticipated Problems policy to review these standards and reporting requirements.
Unanticipated Problems should be reported to irb@uwlax.edu with an Attachment C form.
Guidance on UWL Faculty, Students, and Staff as Participants
Additional Information
IRB Meetings and Deadlines
The IRB Committee is scheduled to meet on the following dates in 2127 Wittich Hall at 8:45 a.m. However, the schedule is subject to change at the discretion of IRB Committee.
9/20/2024; 10/25/2024; 11/15/2024; 12/6/2024
*Protocols that require clinical review are due to irb@uwlax.edu two weeks in advance of the upcoming meeting.
Review Timelines
During the academic year, September to early May:
- Full protocols submitted by the deadlines are expected to be reviewed at the next IRB meeting. Please see the meetings schedule.
- Expedited and exempt protocols are reviewed on an ongoing basis. Review decisions are generally made within three to four weeks of protocol submission.
During the summer, mid-May to August:
- Full protocol review is subject to committee availability. It is not recommended.
- Expedited and exempt protocol review is completed monthly. Review decisions may take up to six weeks following protocol submission.
2024-2025 IRB Committee Members
| Name | Department (College) | Expiration Date | Comments |
| Sandy Grunwald | IRB Administrator | N/A | Ex-officio, non-voting |
| Ward Dobbs | Health Professions (CSH) | August 2025 | First 3-year term |
| Adam Driscoll | Sociology & Criminal Justice (CASSH) | August 2025 | Third 3-year term |
| Shanna Felix | Sociology & Criminal Justice (CASSH) | August 2027 | Second 3-year term |
| Liz Evans | Community Representative (Non-medical) | August 2025 | Second 3-year term |
| Lisa Giddings | Economics (CBA) | August 2026 | Second 3-year term |
| Kari Emineth | Exercise and Sport Science (CSH) | August 2027 | First 3-year term |
| Katherine Kortenkamp | IRB Coordinator, Psychology (CASSH) | N/A | ex-officio, voting |
| Weixu Lu | Communication Studies (CASSH) | August 2027 | Second 3-year term |
| Heidi Morrison | Oral History (CASSH) | August 2026 | First 3-year term |
| Elizabeth Peacock | Archaeology & Anthropology (CASSH) | August 2026 | First 3-year term |
| Drew Rutherford | Health Professions (CSH) | August 2025 | Second 3-year term |
| Jessica Sosso, M.D. | Community Representative (Medical) | August 2026 | First 3-year term |
| Yuko Iwai | Educational Studies (CASSH) | August 2027 | First 3-year term |
Continuing Education and Resources
Best Practices for Conducting Risky Research and Protecting Yourself from Online Harassment
Continuing Education - Fall 2010
IRB Training FAQs
I already completed the NIH Training Course and have a copy of my Completion Certification – will this still be accepted?
- All NIH IRB training certifications will expire December 31, 2023.
- Individuals should complete the appropriate CITI IRB training modules and provide a new complete certificate with their next IRB protocol.
- Only CITI IRB training certificates will be accepted on or after January 1, 2024.
I already completed the NIH Training Course that was previously offered, but don’t have a copy of my Completion Certification or access to my account. Do I need to complete the CITI Training Course?
- Maybe. If you’ve already submitted a copy of your Completion Certification with an IRB protocol in the past at UWL, it’s possible we have record of this. Please email irb@uwlax.edu to inquire if we have your Human Research Subjects Training certification on file.
When does my IRB Completion Certification expire?
- As of September 24, 2018, IRB training expires after a 5-year period.
- For those completing IRB training for the first time in CITI, the required training certification will expire 5 years following an individual's completion date.
- For individuals who completed the old NIH IRB training, certifications will expire December 31, 2023. At that time, individuals should complete the CITI IRB training modules and provide a new completion certification with their next IRB protocol.
How do I get my CITI Program password reset?
- General questions and password reset requests should be addressed to irb@uwlax.edu.
I'm having technical issues with the CITI website - who should I contact?
- Technical issues with the CITI web site should be addressed to support@citiprogram.org or to 888-529-5929.
I have other questions not addressed here about IRB Training - who should I contact?