Tumor burden
A page within IACUC
PURPOSE:
RODENT TUMOR BURDEN POLICY PURPOSE: The purpose of this policy is to provide research staff, investigators and their personnel with clearly defined parameters and mandatory endpoints for the care and use of rodents with experimentally induced or spontaneously developing tumors under study. All principal investigators and laboratory personnel using tumor models are expected to read this policy and operate within established parameters as outlined here. Any deviations from this policy must be explicitly stated and accompanied by scientific justification in an approved IACUC animal care and use protocol.
REFERENCE: Guide for the Care and Use of Laboratory Animals, 8th edition, page 27
DEFINITIONS:
Moribund: Clinically irreversible condition evident in a live animal where death is inevitable.
Ulceration: Tumors developing under the skin surface may devitalize the overlying skin so the overlying skin dies and a wound is created that is initiated from underneath. Ulceration is a lesion typified by necrosis of superficial tissues, which may be dry, suppurating or exudative.
Body Condition Score (BCS): The general physical condition of the animal is the most important factor in effectively following the progression of tumors in rodents. BCS’s are a helpful adjunct to assessment of overall health of the animal.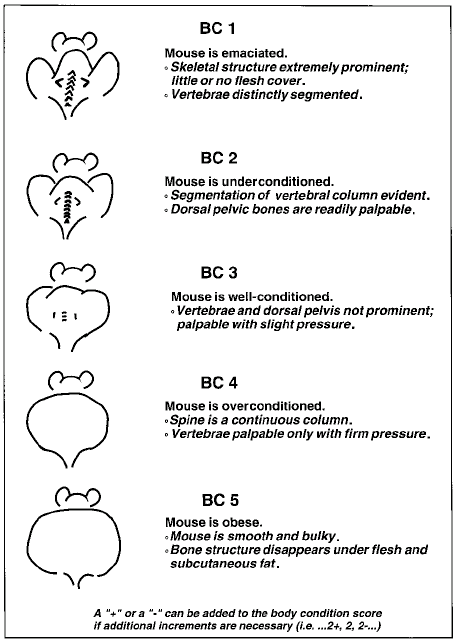
This scoring is also applicable with the rat (3)
POLICY:
All animal use protocols involving tumor cell implantation or production must have a pre-submission veterinary consultation with regard to carefully considered location(s) of tumor cell placement, additional supportive care for animals bearing tumors and humane endpoints.
Tumor cells must be screened for rodent and human pathogens prior to being used in research animals. In addition, human tumor lines require IBC review and approval.
Tumor Size: The visible size of the tumor is only one of the criteria used for the determination of humane endpoints. The overriding consideration for humane endpoints of oncological experiments as well as spontaneous tumors must be the overall health of the animal.
Optimally, one observer should perform all tumor measurements in a given study and calipers must be used to measure tumor size in order to avoid discrepancies. Tumor size must not exceed 20mm (2.0cm) at the largest diameter in mice and 40 mm (4.0cm) in rats. Larger size tumors must be specifically justified in the IACUC protocol. If multiple subcutaneous tumors are implanted, smaller maximum tumor sizes must be described in the IACUC protocol.
Health limitations may be evident before the tumor reaches the maximum standards above. Some limitations may include mobility restriction, the inability to access food and water, pressure on internal organs or sensitive regions of the body, or body condition score (BCS) of < 2. Animals displaying such signs must be euthanized even if the maximum tumor size has not been reached.
Monitoring Schedule:
Prior to tumor inoculations, body weights must be recorded.
Animals that are on a tumor production study must be visually observed and palpated once/weekly during the time when the tumor is not yet detectable until tumor growth has begun.
After a visual or palpable tumor is evident, the animals must be visually monitored daily and BCS and tumor measurements recorded once/weekly. If the BCS falls below three, frequent monitoring of body weights will be required and frequency will be determined by the veterinary staff. More frequent observations may be necessary based on tumor growth rate, study parameters and general condition of the animal.
Clinical Signs Associated with Tumor Progression
1. General Appearance; including dull or closing eyes.
2. Decreased food/water intake.
3. Dehydration.
4. Weight loss (assessed by weighing) and/or Body Condition Score (BCS).
5. Depressed or restless activity or abnormal aggression.
6. Respiratory difficulty.
7. Cranial deformity/neurological signs.
8. Rough hair coat and/or hunched posture.
9. Restricted mobility.
10. Changes in feces/urine and/or perianal soiling.
11. Eye and nose porphyrin (red stain) discharge.
12. Abdominal distention (evidence for accumulation of hemorrhagic ascites or large internal tumor).
Records must be kept and be available in the animal room with all pertinent information including time and frequency of monitoring sessions, the name of the person monitoring the animals, identification of the animals, protocol number, the number of animals displaying symptoms, types of symptoms, and any treatments given to the animals.
Endpoints: The overall well-being of the animal takes priority over precise tumor measurements in decisions regarding euthanasia or other interventions. Tumors induced in body cavities (cranium, orbit, abdomen, or thorax) may have additional limitations as to the maximum acceptable size. These animals must be monitored very closely for any severe impairment in physiological or neurological function and be euthanized as soon as such signs become apparent.
The following clinical signs are indications of morbidity. Tumor-bearing animals exhibiting any of these signs should be euthanized based on severity of clinical signs:
1. Tumor size exceeds 20mm (2.0cm) at the largest diameter in mice or 40mm (4.0cm) in rats.
2. Ulcerated/necrotic tumor resulting in skin breakdown or exudation persisting beyond 48 hours.
3. Tumor interferes with normal bodily functions (i.e., ambulation, eating, drinking, defecation, and urination).
4. The tumor affects the rodent’s gait or normal posture, independent of the size of the tumor.
5. Labored breathing.
6. Lack of movement.
7. Hypothermia (cool to the touch).
8. Self-mutilation.
9. The veterinary staff determines that the animal should be euthanized for humane concerns.
Adoption Date: 5/10/16
Amended:
Reference minutes: 5/10/16


