Institutional Biosafety Committee (IBC)
A page within Research & Sponsored Programs
The Institutional Biosafety Committee (IBC) has oversight of research using biological materials that entail a potential risk to humans, animals, or the environment. To fulfill this commitment, the IBC reviews and monitors activities involving materials such as recombinant or synthetic nucleic acid molecules; microorganisms and viruses; infectious agents or pathogens; biological toxins; human-derived tissues, fluids, and cells; non-human animal-derived tissues, fluids, and cells that are infectious, potentially infectious, or recombinant; genetically modified live animals; or other biological materials that may be toxic to living organisms. The IBC ensures compliance with the NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules and other biosafety requirements, such as the CDC Biosafety in Microbiological and Biomedical Laboratories (BMBL). Roles and responsibilities, biosafety requirements and best practices, and general biosafety information can be found in the UWL Biosafety Manual.
Forms
Form List & When to Submit
Submit all completed forms and attachments via email to grants@uwlax.edu.
| Form | When to Submit |
| Biosafety Protocol Application |
Submit for:
|
| Project Inclusion of DURC & PEPP |
Submit when you are including Dual Use Research Concern (DURC) and/or Pathogens with Enhanced Pandemic Potential (PEPP) substances in your new IBC protocol |
| DURC & PEPP Progress Report | Submit to re-certify use of DURC and/or PEPP substances in your approved IBC protocol |
| IBC Personnel & Award Modification Form | Submit to modify an existing protocol to add/remove personnel or add associated external awards. |
| IBC Protocol Closure Form | Submit once a project with an approved protocol has been completed. |
| IBC Incident Report Form |
Submit within 24 hours of any spill or accident involving recombinant material research or that otherwise leads to personal injury or illness or to a breach of containment. The following types of incidents must be reported to the NIH Office of Science Policy (OSP) immediately:
For more information, see NIH incident reporting FAQs. |
| Biosafety Noncompliance Reporting Form (Anonymous) | Submit concerns about biosafety noncompliance anonymously. See the UWL IBC Noncompliance Policy for additional information. |
When Is IBC Review Required?
Activities Requiring IBC Review
If research involves any biological materials that entail a potential risk to humans, animals, or the environment, a Biosafety Protocol Application needs to be completed. This includes work with materials that are not subject to the NIH Guidelines or that are exempt under the NIH Guidelines, Section III-F. The table below provides an overview of materials requiring the submission of a Biosafety Protocol Application. Submit the completed protocol form and attachments via email to grants@uwlax.edu.
|
Area |
Description |
|
Recombinant or synthetic nucleic acid molecules |
Recombinant materials, including those that are chemically or otherwise modified but can base pair with naturally occurring nucleic acid molecules, or cells, organisms, and viruses containing such molecules. |
|
Microorganisms and viruses |
Agents associated with human disease that pose moderate hazards to personnel and the environment. Microorganisms include bacteria, protozoa, algae, and fungi. Although viruses are not considered living organisms, they are included in this classification. |
|
Prions |
Prions are abnormal, pathogenic proteins that are transmissible and are able to induce abnormal folding of specific normal cellular proteins called prion proteins that are found most abundantly in the brain. |
|
Non-human animal tissues, cell lines, or blood products |
All cell and organ cultures and materials of non-human animal origin that are infectious, potentially infectious, or recombinant. |
|
Human cells and cell culture, organs or tissues, or biological samples |
All cell and organ cultures and materials of human origin. |
|
Genetically modified live animals |
Animals (vertebrate and/or invertebrate) that are recombinant (transgenic), exotic, and/or grown in association with pathogens and/or recombinant materials. Research involving recombinant DNA introduced into vertebrate animals will require both IBC and IACUC approval. |
|
Plants and soils |
Plants that are recombinant (transgenic), exotic, and/or grown in association with pathogenic or recombinant microbes and/or pathogenic or recombinant small animals (insects, etc.). Research involving soil, seed, plants, plant pathogens, or other materials as regulated by state or federal policy or law. Samples from a general population that is not considered to be at an increased risk for contamination with pathogens do not need a Biosafety Protocol. Samples that may be at an increased risk for contamination with pathogens do require a Biosafety Protocol. |
|
Biological toxins |
Biological toxins are poisonous substances produced by certain microorganisms, animals, and plants; this includes protein toxins and low molecular weight toxins, as described in the CDC BMBL, Section VIII-G. This does not include toxic chemicals or antibiotics, which instead require Chemical Hygiene Plan review and approval by Environmental Health & Safety. |
|
Dual Use Research of Concern (DURC) |
DURC is life sciences research that can be reasonably anticipated to generate knowledge, information, technologies, and/or products that could be utilized for both benevolent and harmful purposes and could pose a significant threat with broad potential consequences to public health and safety, agricultural crops or other plants, animals, the environment, or national security. See Dual Use Research of Concern (DURC). |
|
Select Agents and Toxins |
Biological Select Agents and Toxins (BSATs) are biological agents that have been declared by the US Department of Health and Human Services (HHS) or by the US Department of Agriculture (USDA) to have the “potential to pose severe threat to public health and safety.” See www.selectagents.gov. |
Submission Types & Review Process
Submission Types
Submit all completed forms and attachments via email to grants@uwlax.edu.
| Submission Type | When to Submit | What to Submit |
| New biosafety protocol | To obtain initial IBC approval for new projects |
|
| Inclusion of Dual Use Research of Concern (DURC) and/or Pathogens with Enhanced Pandemic Potential (PEPP) components of protocol | To obtain initial IBC approval and continued IBC approval for the inclusion of DURC and/or PEPP on projects | |
| Renewal of biosafety protocol | To renew a previously approved protocol after 3 years to extend IBC approval of ongoing work for another 3-year period |
|
| Revision to biosafety protocol | To submit changes for a previously approved protocol related to research elements, biological materials used, and/or location(s) |
|
| Personnel & award modifications | To add or remove personnel, or add associated external awards, to a previously approved protocol |
|
| Protocol closure | To close an approved protocol once a project has been completed |
Protocol Review Process
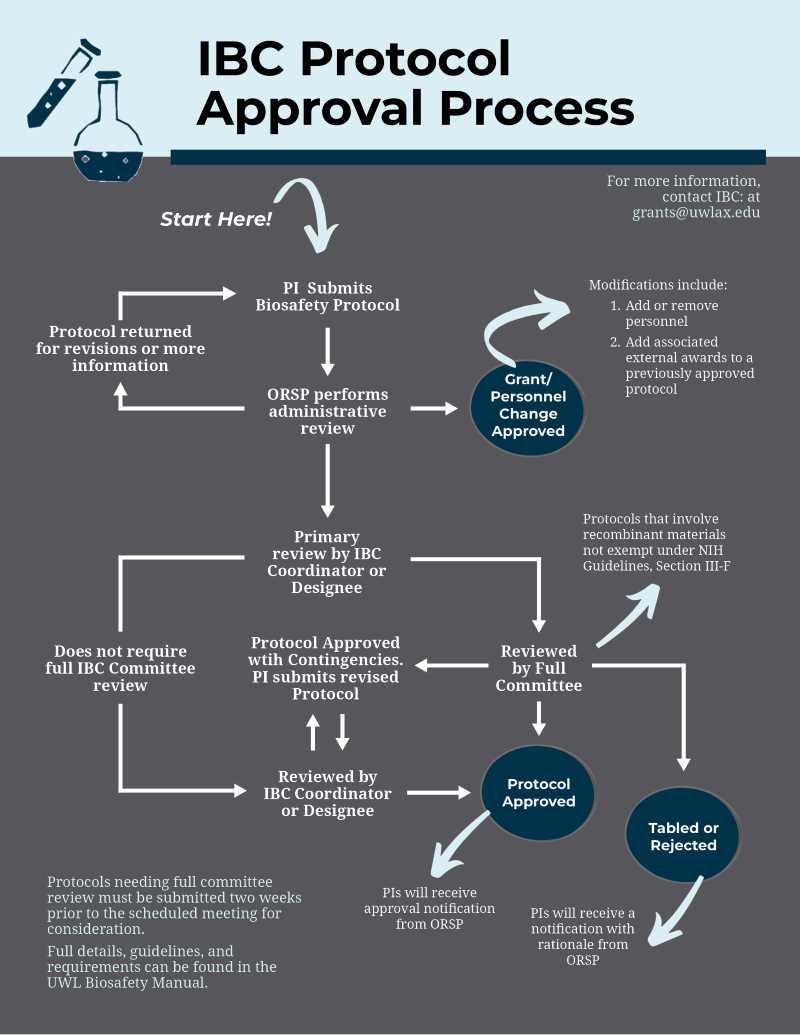 IBC Protocol Review Process Flowchart
IBC Protocol Review Process Flowchart
Review Timelines
During the academic year (September to early May):
- Protocols requiring review by the full committee submitted at least two weeks prior to an upcoming IBC meeting are expected to be reviewed at the next IBC meeting. Please see the meetings schedule.
- Protocols not requiring review by the full committee are reviewed on an ongoing basis. Review decisions are generally made within three to four weeks of protocol submission.
During the summer (mid-May to August):
- The IBC does not meet June-August. Protocols requiring full committee review are expected to be reviewed at the first IBC meeting of the following academic year.
- Protocols not requiring full committee review are reviewed monthly. Review decisions may take up to six weeks following protocol submission.
Research involving recombinant or synthetic nucleic acid molecules at Biosafety Level 3 (BSL3) or higher or in large-scale (greater than 10 liters), or research involving gene drive modified organisms (GDMOs) requires the university to have a designated Biological Safety Officer (BSO). UWL currently does not have a designated BSO. Consequently, investigators proposing such research should be aware that there will be an extended timeline needed for the university to prepare to meet the additional requirements before the research can be conducted.
Biosafety Protocol Form Examples
Recombinant Materials - Exempt
- Biosafety Protocol Form Example - recombinant materials exempt under NIH Guidelines Section III-F, microorganisms
- Biosafety Protocol Form Example - recombinant materials exempt under NIH Guidelines Section III-F, microorganisms
Recombinant Materials - Full Review
Training
Required Training Modules
Training is provided online through CITI, and basic IBC training is valid for three years. All faculty, staff, and students listed in a Biosafety Protocol Application must complete the Basic IBC Training Modules designated below. Additional training requirements may apply based on research type, which are listed in the second table below. The minimum passing aggregate score for quizzes is 80%, which is automatically tracked by CITI. CITI training completion certificates must be attached to the Biosafety Protocol Application for all personnel listed in the protocol.
Basic IBC Training Requirements
|
Course |
Faculty & Staff |
Students |
|
Biosafety Course Overview (13314) |
Required |
Required |
|
Laboratory-Acquired Infections (13454) |
Required |
Required |
|
Biohazard Risk Assessment (13455) |
Required |
Optional |
|
Medical Surveillance (13456) |
Required |
Optional |
|
Risk Management: Work Practices (13898) |
Required |
Required |
|
Risk Management: Personal Protective Equipment (13458) |
Required |
Required |
|
Risk Management: Emergency and Spill Response (13459) |
Required |
Required |
|
Risk Management: Engineering Controls (13929) |
Required |
Optional |
|
Work Safely with Sharp Instruments (13899) |
Required |
Required |
|
Disinfection and Sterilization (13900) |
Required |
Required |
|
Centrifuge Precautions (13945) |
Required |
Optional |
Additional Training Requirements by Research Type
|
Research |
Additional Course(s) Required |
|
Research involving recombinant DNA, recombinant RNA, or synthetic nucleic acids |
NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (13493) |
|
Research involving human-derived materials, cell lines, and/or bloodborne pathogens |
Contact UWL Environmental Health & Safety for initial and annual training requirements. |
|
Research involving vertebrate or invertebrate animals |
Animal Biosafety (13654) Additional IACUC training requirements apply for vertebrate animals. See IACUC guidelines. |
|
Research involving nanotechnology |
Understanding Nanotechnology and Its Implications (14044) |
|
Research involving select agents |
|
|
Research involving Dual Use Research of Concern (DURC) |
Dual Use Research of Concern (DURC) (16263) |
|
Research involving plants and/or soils |
USDA Permits:
|
|
Research involving human gene transfer |
Human Gene Transfer Research (13494) |
How to Register for & Enroll in CITI Training
Note: If you already have a CITI account and just need to add the relevant Biosafety/Biosecurity training modules, log-in to CITI and select "Add a Course." Skip to Step 7 of the instructions below to enroll in Biosafety/Biosecurity training.
Step 1: Go to the CITI registration page. Under “Select Your Organization Affiliation”, type “Wisconsin” in the box. Select University of Wisconsin-La Crosse. Click on “Continue to Create Your CITI Program Username/Password."
Step 2: Enter your first name, last name, and email address. Enter your name here as you would like it to appear on your training completion reports. Please note that using your UWL-issued email address is preferred for account recovery purposes. Click on “Continue to Step 3.”
Step 3: Choose a user name, password, and security question. Click on “Continue to Step 4.”
Step 4: Enter demographic information and click on “Continue to Step 5.”
Step 5: Click “NO” when asked if you want to request Continuing Education Unit (CEU) credits. Answer the next two questions and click on “Continue to Step 6.”
Step 6: Complete the personal information requested. For your Department, students can use their major (Biology, Microbiology, etc.). For the Role in Research, choose a role that is most appropriate for your involvement in the research. Click on “Continue to Step 7”.
Step 7: Scroll down to Question 10 (SKIP all other questions), "Biosafety/Biosecurity," to select the appropriate curriculum. Select ALL relevant learner groups based on your project role and the type of research activities you will conduct. Students should consult with their faculty advisor to ensure they are selecting the appropriate group(s):
- Faculty & Staff: ALL faculty & staff should select this to satisfy basic IBC training requirements.
- Faculty/Students Working with DURC Agents: Faculty, staff, and students working with Dual Use Research of Concern (DURC) agents should select this to complete the additional module required for this type of research.
- Students: ALL students should select this to satisfy basic IBC training requirements.
- IBC Committee Members: This provides training resources for IBC committee members.
- RIO, IBC Chair, Lab Managers, ORSP Staff: This provides training resources for the Research Integrity Officer (RIO), IBC Chair, lab managers, and Office of Research & Sponsored Programs (ORSP) staff.
- BSO: This provides training resources for the Biological Safety Officer (BSO).
- Faculty/Students Working with Recombinant DNA/RNA: All faculty, staff, and students working with recombinant DNA/RNA or synthetic nucleic acids should select this to complete the additional training module required for this type of research.
- Faculty/Students Working with Human-Derived Materials and/or Bloodborne Pathogens: This provides training resources but does NOT fulfill the annual training requirements for research involving human-derived materials, cell lines, and/or bloodborne pathogens. Contact UWL Environmental Health & Safety to complete required training.
- Faculty/Students Working with Nanotechnology: All faculty, staff, and students working with nanotechnology should select this to complete the additional training module required for this type of research.
- Faculty/Staff Shipping Regulated Biological Materials: This provides training resources for faculty and staff planning to ship biological materials.
- Faculty/Students Working with Vertebrate or Invertebrate Animals: All faculty, staff, and students working with vertebrate or invertebrate animals should select this to complete the additional training module required for this type of research.
- Faculty/Students Working with Select Agents: All faculty, staff, and students working with select agents should select this to complete the three additional training modules required for this type of research.
- Faculty/Students Working with Plants or Soils: All faculty, staff, and students working with plants and/or soils should select this to complete the three additional training modules required for this type of research.
Step 8: Click on “Submit” at the bottom of the page.
How to Complete Required IBC Training
Step 1: Log into your CITI account. From your list of courses, select the Biosafety/Biosecurity course(s) you have recently added to begin.
Step 2: Complete the Integrity Assurance Statement presented at the top of each course. The system will allow you to start taking the course modules after completing it.
Step 3: Click the title of each course to begin or continue the course.
Step 4: Complete the required modules and associated quizzes. Please read the course main page for requirements, as there may be elective and/or required modules based on your selected course. Supplemental modules are optional, unless assigned by a professor, supervisor, or supervising investigator, or required of you by the IBC due to the nature of your project.
The minimum passing aggregate score for the quizzes is 80%. A running tally is compiled in the CITI grade book. If you want to improve a score on a quiz, you may repeat any quiz. Scores obtained after a completion report has been issued will not be reflected on the completion report.
Step 5: When you complete all required modules for a course successfully, a Completion Certificate will be generated. The IBC will need a copy of this document included in the submission of a Biosafety Protocol Application for all individuals listed in the protocol. You can access the Completion Certificate under the “My Records” tab.
Find the course you have just completed, and click “View-Print-Share” under "Completion Record." There will be two options for completion records – the document needed can be found by clicking "View/Print" under Completion Certificate.
Step 6: Save this Completion Certificate as a PDF or Word document. Send a copy to the faculty member serving as the Principal Investigator (PI) to submit with the Biosafety Protocol Application.
General questions and password reset requests should be addressed to grants@uwlax.edu.
Technical issues with the CITI website should be addressed to support@citiprogram.org or 888.529.5929.
Biosafety Incidents & Noncompliance
Report Biosafety Incident or Noncompliance Concerns
| Form | When to Submit |
| IBC Incident Report Form |
Submit within 24 hours of any spill or accident involving recombinant material research or that otherwise leads to personal injury or illness or to a breach of containment. Submit completed form via email to grants@uwlax.edu. The following types of incidents must be reported to the NIH Office of Science Policy (OSP) immediately:
For more information, see NIH incident reporting FAQs. |
| Biosafety Noncompliance Reporting Form (Anonymous) | Submit concerns about biosafety noncompliance anonymously. See the UWL IBC Noncompliance Policy for additional information. |
IBC Noncompliance Policy
UWL defines noncompliance as any failure to follow (1) federal regulations, state laws, or institutional policies relevant to biosafety, or (2) the requirements and determinations of the reviewing IBC. To report what you believe to be an instance of noncompliance related to research conducted with biological materials, or if you have concerns that your own research may not be in compliance, please contact the Office of Research & Sponsored Programs (grants@uwlax.edu) or submit an anonymous report.
Biosafety Incidents, Violations, Accidents, & Illnesses
In accordance with the NIH Guidelines, significant problems with or violations of the NIH Guidelines and any significant research-related accidents or illnesses will be reported to the NIH Office of Science Policy (OSP) within thirty days or immediately depending on the nature of the incident. PIs are responsible for reporting incidents within 24 hours by submitting an IBC Incident Report Form to grants@uwlax.edu. Reports will be submitted to the NIH OSP by the Associate Vice Chancellor for Academic Affairs/Research Integrity Officer (RIO). Additional information about incident reporting requirements can be found in the NIH incident reporting FAQs.
The following types of incidents must be reported to OSP immediately:
- Any spills or accidents in BSL-2 laboratories resulting in an overt exposure or
- Spills or accidents occurring in high containment (BSL-3 or BSL-4) laboratories resulting in overt or potential exposure
Any spill or accident involving recombinant DNA or synthetic nucleic acid molecules research of the nature described above or that otherwise leads to personal injury or illness or to a breach of containment must be reported to OSP. These kinds of events might include skin punctures with needles containing recombinant DNA or synthetic nucleic acid molecules, the escape or improper disposition of a transgenic animal, or spills of high-risk recombinant materials occurring outside of a biosafety cabinet. Failure to adhere to the containment and biosafety practices articulated in the NIH Guidelines must also be reported to OSP.
Minor spills of low-risk agents not involving a breach of containment that were properly cleaned and decontaminated generally do not need to be reported. OSP should be consulted if the IBC is uncertain whether the nature or severity of the incident warrants reporting; OSP can assist in making this determination.
IBC Members & Meeting Information
IBC Member Roster
Basudeb Bhattacharyya, PhD
Sierra Colavito, PhD
Sandy Grunwald, PhD (Research Integrity Officer)
Sumei Liu, PhD (IACUC & IBC Coordinator)
John May, PhD
Todd Osmundson, PhD
William Schwan, PhD
Paul Schweiger, PhD (Chair)
Community Members
Jay Ellingson, PhD
Arick Sabin, DO
Kim Fredricks, PhD
IBC Meetings & Submission Deadlines
The IBC Committee is scheduled to meet on the following dates. The schedule is subject to change at the discretion of IBC Committee. All meetings will take place in 228 Graff Main Hall.
Protocols that require full committee review must be submitted at least two weeks prior to an upcoming IBC meeting. IBC does not meet in January or June-August. Plan submissions requiring full committee review accordingly.
| IBC Meeting Date | Submission Deadline for Protocols Requiring Full Review |
| February 26, 2024, 9:55 am | February 12, 2024 |
| March 25, 2024, 9:55 am | March 11, 2024 |
| April 11, 2024, 9:00 am | March 28, 2024 |
| May 2, 2024, 9:00 am | April 18, 2024 |
IBC Operating Guidelines & Policies
IBC Meeting Minutes
Resources
IBC Resources
- UWL Biosafety Manual
- NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules
- CDC Biosafety in Microbiological and Biomedical Laboratories (BMBL)
- Biological Risk Assessment, CDC
- Risk Group (RG) Database, American Biological Safety Association (ABSA)
Biosafety Manual Link Resources
TBD


